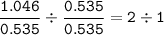The empirical formula of the compound : Cu₂O
Further explanation
Given
66.41 g Cu
8.56 g O
Required
The empirical formula
Solution
1. Find mol
mol = mass : Ar
mol = 66.41 : 63.5
mol = 1.046
mol = 8.56 : 16
mol = 0.535
2. mol ratio
Cu : O (divide by smallest mol⇒0.535) :