Answer:
The
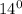 sample water would be more dense.
sample water would be more dense.
Step-by-step explanation:
As it has been proved that the density of a water depends on its salinity and its temperature. The cold water is denser and it will be less dense when it is warm. The density is dependent on the salinity too.
So, it is evident that among the two mentioned samples the sample B with the temperature of 14 degree will be denser than the sample of salty water that has 25 degree temperature.