The oxidation number of Mn in
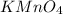 is +7.
is +7.
Step-by-step explanation:
In a compound the sum of the oxidation states of all the atoms will be to equal the total charge on the ions.
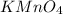 is neutral hence its total charge is zero
is neutral hence its total charge is zero
Oxidation number of potassium+ Oxidation number of Manganese+Oxidation number of Oxygen=0
We Know that
Oxidation number of potassium= +1
Oxidation number of Oxygen= -2
Substituting the values,
(+1)+ Oxidation number of Manganese+ 4(-2)=0
(+1)+ Oxidation number of Manganese+ (-8)=0
(-7)+ Oxidation number of Manganese=0
Oxidation number of Manganese= +7