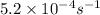Answer:
Rate constant at 725 K is
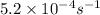
Step-by-step explanation:
According to Arrhenius equation for a reaction-
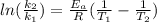
where
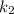 and
and
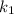 are rate constants of reaction at
are rate constants of reaction at
 and
and
 temperatures (in kelvin) respectively.
temperatures (in kelvin) respectively.
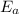 is activation energy of reaction.
is activation energy of reaction.
Here
 = 600 K ,
= 600 K ,
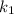 =
=
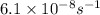
 = 725 K,
= 725 K,
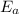 = 262 kJ/mol and R = 8.314 J/(mol.K)
= 262 kJ/mol and R = 8.314 J/(mol.K)
So plugin all the values in the above equation-
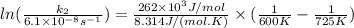
So,
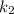 =
=
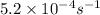
Hence rate constant at 725 K is