Magnesium and fluorine atoms most likely form an ionic bond.
Step-by-step explanation:
An ionic bond is formed when a metal and a nonmetal react. There is a transfer of electrons. Magnesium has a charge of 2+ and Fluorine has a charge of -1so by transferring of electrons it becomes
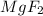 And we know magnesium is a metal and fluorine is a non metal. When they both react, they form an ionic bond.
And we know magnesium is a metal and fluorine is a non metal. When they both react, they form an ionic bond.
Whereas in covalent bond, there is a sharing of electrons and it is most of the time between two nonmetals.