The theoretical yield : 31.11 g
Further explanation
Given
Reaction
3H2+N2=2NH3
Required
the theoretical yield
Solution
mol = mass : Ar
mol = 5.5 g : 2
mol = 2.75
From the equation, mol ratio of H₂ : NH₃ = 3 : 2, so mol NH₃ =
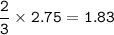
- Mass Ammonia (MW=17 g/mol):
mass =mol x MW
mass = 1.83 x 17
mass= 31.11 g