Answer:
heat transfer is 4.6067 kJ
Step-by-step explanation:
given data
P1= 2 bar = 2 ×
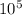 N/m²
N/m²
P2= 8 bar = 8 ×
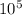 N/m²
N/m²
V2= 0.02 m³
m = 0.2 kg
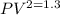 = constant
= constant
V(1 to 2) = 50 kJ/kg
U(1 to 2) = m V(1 to 2) = 0.2 × 50 = 10 kJ
solution
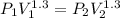
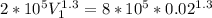
v1 = 0.05809 m³
and
by 1st law of thermodynamics for closed system is
Q - W = ΔU
so calculate the work expansion that is
W =
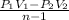
W =
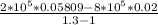
W = -14.6067 kJ
so heat transfer from 1st law of thermodynamic is
Q = U + W
Q = 10 + ( - 14.06067 )
Q = -4.6067kJ
so
heat transfer is 4.6067 kJ