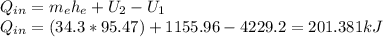Answer:
201.381kJ
Step-by-step explanation:
We have here a mixed fluid, so initially
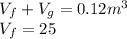
From saturated tables, at 800kPa
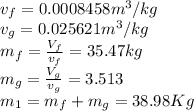
We know that at the end, the Volume is only the initial vapor,
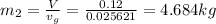
From the tables at 800kPA
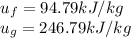
So,
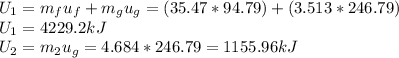
The exiting fluid is saturated, so the equation that we have is
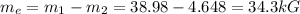
We search in the tables at 800kPa
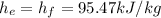
We can make a energy balance,