Answer:
2 L
Explanation:
At constant pressure, the volume of a gas is proportional to its temperature.
Therefore, we can write
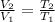 ....... (1) at pressure = constant.
....... (1) at pressure = constant.
Given that the initial volume (
 ) = 2.5 L and initial temperature (
) = 2.5 L and initial temperature (
 ) = 350 K
) = 350 K
And the final temperature (
 ) = 280 K
) = 280 K
Therefore, from equation (1) we get final volume (
 ) =
) =
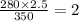 L (Answer)
L (Answer)