Answer:
For a: The increasing of atomic radius is:
Al < Mg < Na
For b: The pH of the solution of sodium oxide is greater than 7.
Step-by-step explanation:
Atomic radius is defined as the distance of the nucleus to the outermost shell containing electrons.
It decreases as we move from left to right in a period because electrons get added up in the same shell and effective nuclear charge increased which results in the shrinkage of the atom.
Sodium lies in Period 3, group 1. Its atomic radius is 1.90
Magnesium lies in Period 3, group 2. Its atomic radius is 1.60
Aluminium lies in Period 3, group 3. Its atomic radius is 1.32
The increasing of atomic radius will be:
Al < Mg < Na
The mathematical values proves the result.
There are 3 types of solution based on pH:
- If pH > 7, the solution is basic in nature.
- If pH < 7, the solution is acidic in nature.
- If pH = 7, the solution is neutral in nature.
When a metal reacts with oxygen present in air, it forms basic oxide which simply means when they react with water, they form basic solution.
The chemical equation for the reaction of sodium (metal) with oxygen present in air and reaction with water of the product so formed are as follows:
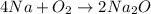
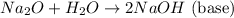
Sodium forms a basic solution when its oxide reacts with water, therefore the pH of the solution will be greater than 7.