Answer:
0.96kg/s
Step-by-step explanation:
Hello! To solve this exercise we must use the first law of thermodynamics, which states that the sum of the energies that enter a system is the same amount that must go out. We must consider the following!
state 1 : is the first flow in the input of the chamber
h1=entalpy=335.02KJ/kg
m1=mass flow=0.56kg/s
state 2 : is the second flow in the input of the chamber
h2=entalpy=83.915KJ/kg
state 3:is the flow that comes out
h3=entalpy=175.90 kJ/kg
now use the continuity equation that states that the mass flow that enters is the same as the one that comes out
m1+m2=m3
now we use the first law of thermodynamics
m1h1+m2h2=m3h3
335.02m1+83.915m2=175.9m3
as the objective is to find the cold water mass flow(m2) we divide this equation by 175.9
1.9m1+0.477m2=m3
now we subtract the equations found in the equation of continuity and first law of thermodynamics
m1 + m2 = m3
-
1.9m1 + 0.477m2=m3
----------------------------------
-0.9m1+0.523m2=0
solving for m2
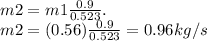
the mass flow rate of the cold-water is 0.96kg/s