The question is incomplete, here is the complete question:
The half-life of a certain radioactive substance is 46 days. There are 12.6 g present initially.
When will there be less than 1 g remaining?
Answer: The time required for a radioactive substance to remain less than 1 gram is 168.27 days.
Explanation:
All radioactive decay processes follow first order reaction.
To calculate the rate constant by given half life of the reaction, we use the equation:
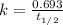
where,
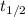 = half life period of the reaction = 46 days
= half life period of the reaction = 46 days
k = rate constant = ?
Putting values in above equation, we get:
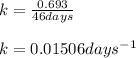
The formula used to calculate the time period for a first order reaction follows:
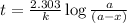
where,
k = rate constant =
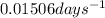
t = time period = ? days
a = initial concentration of the reactant = 12.6 g
a - x = concentration of reactant left after time 't' = 1 g
Putting values in above equation, we get:
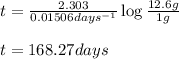
Hence, the time required for a radioactive substance to remain less than 1 gram is 168.27 days.