Answer:
5 L , 10 L and 30 L of each solution should be used respectively.
Step-by-step explanation:
Volume of solution-1 = x
Volume of solution-2 = y
Volume of solution-3 = z
Total volume of an acid solution desired = 45 L
x + y + z = 45 L
Percentage of an acid in solution-1 = 20%
Volume of an acid in solution-1 = 20% of x = 0.2x
Percentage of an acid in solution-2 = 35%
Volume of an acid in solution-2 = 35% of y = 0.35y
Percentage of an acid in solution-3 = 75%
Volume of an acid in solution-3 = 75% of z = 0.75z
Percentage of an acid solution desired = 60%
Volume of an acid in desired solution =
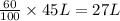
Sum of volume of acids in all three solution will be equal to 27 L.
0.2x + 0.35y + 0.75z = 27 L
Also give that, chemist want prepare solution by using 3 times as much of the 75 % solution as the 35 % solution.
3y = z
x + y + z = 45 L
x + 4y = 45 L..[1]
0.2x + 0.35y + 0.75z = 27 L
0.2x + 0.35y + 0.75(3y) = 27 L
0.2x + 2.6y= 27 L...[2]
On solving [1] and [2] ,we get:
x = 5 L
y = 10 L
z = 3y = 30 L
5 L , 10 L and 30 L liters of each solution should be used respectively.