Answer:
She is not getting a good deal.
Step-by-step explanation:
The equation that relates heat with mass, specific heat and temperature change of an object is
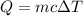 .
.
Always convert temperature to Kelvin, although in our case it's not necessary because the
 will be the same, and we will leave the mass in grams because we will be getting
will be the same, and we will leave the mass in grams because we will be getting
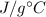 units for specific heat, which we can compare to the one given for gold.
units for specific heat, which we can compare to the one given for gold.
We then calculate the specific heat of the object in question:
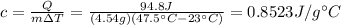
Which shows it's not gold.