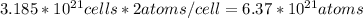Answer:
(a)
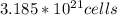
(b)
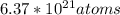
Step-by-step explanation:
(a)
Volume, V of unit cell
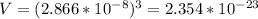
Number of unit cells, N
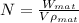 Where
Where
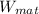 is weight of material and
is weight of material and
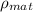 is density of material
is density of material
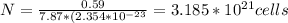
(b)
Number of atoms in paper clip
This is a product of number of unit cells and number of atoms per cell
Since iron has 2 atoms per cell
Number of atoms of iron=