Answer:
MM = 156.57 g/mol
pH at V = 9.5 mL will be the pKa value for the acid
Step-by-step explanation:
solution
for the molar mass MM is here express as
MM =
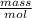 .....................1
.....................1
here
mass = 0.2975 g
and
mol = MV = 0.1000 × 19 ×
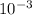
MV = 0.0019 mol of acid
so
MM =
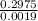
MM = 156.57 g/mol
and
we know for pKa the easiest way is to find the half equivalence point is
V half = 0.5 × Vtitration
V half = 0.5 × 19
V half = 9.5 mL
so pH at V = 9.5 mL will be the pKa value for the acid