Answer: The mass percent of
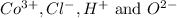 ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively
ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively
Step-by-step explanation:
We are given:
Mass of silver chloride = 0.308 grams
Mass of cobalt (III) oxide = 0.145 grams
Mass of sample containing chloride ions = 0.256 grams
Mass of sample containing
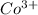 ions = 0.416 grams
ions = 0.416 grams
We know that:
Molar mass of silver chloride = 143.4 g/mol
Molar mass of chlorine ion = 35.45 g/mol
Molar mass of cobalt (III) oxide = 165.86 g/mol
Molar mass of
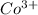 ion = 117.86 g/mol
ion = 117.86 g/mol
Molar mass of water = 18 g/mol
Molar mass of hydrogen ion = 1 g/mol
Molar mass of oxygen ion = 16 g/mol
To calculate the number of moles, we use the equation:
 ......(1)
......(1)
To calculate the mass percentage of ions in sample, we use the equation:
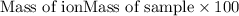 .......(2)
.......(2)
Calculating moles of AgCl by using equation 1:
Moles of AgCl =
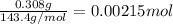
The chemical equation for the ionization of silver chloride follows:
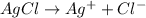
By Stoichiometry of the reaction:
1 mole of silver chloride produces 1 mole of chlorine ions
So, 0.00215 moles of silver chloride will produce =
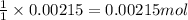 of chloride ions
of chloride ions
Mass of chloride ions =
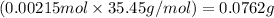
Putting values in equation 2, we get:
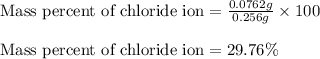
Mass percent of chloride ion = 29.76 %
- For
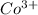 ion:
ion:
Calculating moles of cobalt (III) oxide by using equation 1:
Moles of cobalt (III) oxide =
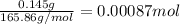
The chemical equation for the ionization of cobalt (III) oxide follows:
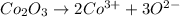
By Stoichiometry of the reaction:
1 mole of cobalt (III) oxide produces 2 moles of cobalt ions
So, 0.00087 moles of cobalt (III) oxide will produce =
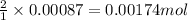 of cobalt ions
of cobalt ions
Mass of cobalt ions =
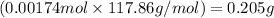
Putting values in equation 2, we get:
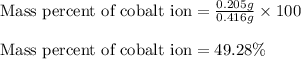
Mass percent of cobalt ion = 49.28 %
- For hydrogen and oxygen ion:
Calculating mass percent of water:
Mass percent of water = 100 - (29.76 + 49.28) = 20.96 %
Let the mass of compound be 100 grams. So, mass of water in the compound will be 20.96 g
For hydrogen:
In 18 grams of water, mass of hydrogen ion present is (1 × 2) = 2 g
So, in 20.96 grams of water, mass of hydrogen ion present will be =
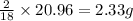
Putting values in equation 2, we get:
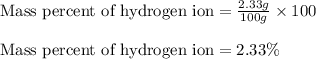
Mass percent of hydrogen ion = 2.33 %
For oxygen:
In 18 grams of water, mass of oxygen ion present is (16 × 1) = 16 g
So, in 20.96 grams of water, mass of oxygen ion present will be =
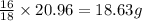
Putting values in equation 2, we get:
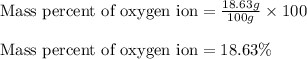
Mass percent of oxygen ion = 18.63 %
Hence, the mass percent of
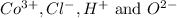 ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively
ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively