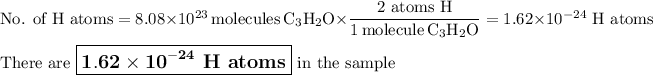Answer:
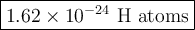
Step-by-step explanation:
1. Calculate the moles of C₃H₂O
Moles of C₃H₂O = 72.5 g C₃H₂O × (1 mol C₃H₂O /54.05 g C₃H₂O)
= 1.341 mol C₃H₂O
2. Calculate the molecules of C₃H₂O
6.022 × 10²³ molecules C₃H₂O = 1 mol C₃H₂O
Molecules of C₃H₂O
= 1.341 mol C₃H₂O × (6.022 × 10²³ molecules C₃H₂O /1 mol C₃H₂O)
= 8.08 × 10²³ molecules C₃H₂O
3. Calculate the number of H atoms.
There are two H atoms in one molecule of C₃H₂O