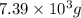Answer:
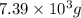 of acetic anhydride is needed
of acetic anhydride is needed
Step-by-step explanation:
According to balanced equation, 1 mol of salicylic acid completely reacts with 1 mol of acetic anhydride
Number of moles = (mass)/(molar mass)
Molar mass of acetic anhydride = 102.09 g/mol
Molar mass of salicylic acid = 138.121 g/mol
So,
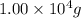 of salicylic acid =
of salicylic acid =
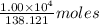 of salicylic acid
of salicylic acid
Hence,
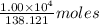 of salicylic acid reacts completely with
of salicylic acid reacts completely with
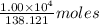 of acetic anhydride.
of acetic anhydride.
So, mass of acetic anhydride needed =
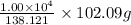 =
=