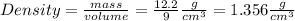Answer:
Check for a possible typo in your text, and pick the answer appropriately as explained below:
The cube's density is either
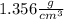 , or
, or
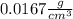
Step-by-step explanation:
There is something incoherent with the dimensions shown in the question: The length of an abject cannot be given in cubic centimeter. It could be just centimeter (cm). Now, if you typed "length" by error, and the word in the question was in fact "volume", the units would be correct.
I am writing below the answers for either case, but please check what the correct enunciation of the problem was:
A) In the case in which the problem stated: If the mass of a cube with a length of 9.0 cm is 12.2 g, what is its density?
The steps are: 1) Recall that the formula for density states that it is the mass of the object divided its volume. The mass is given to you, but only the length of a side of the cube is the info, so you need to find the cube's volume by multiplying 9cm * 9cm *9cm = 729
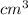 Thus, the density is calculated as the quotient:
Thus, the density is calculated as the quotient:
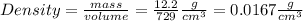
B) In the case the info shown is a volume and not a length, then you don't need to evaluate the volume of the cube (it is already given to you as 9
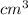 ), and you use it directly in the equation:
), and you use it directly in the equation: