Answer : The concentration of nitrogen gas and hydrogen gas that reacted initially are 10.0 M and 11.0 M respectively.
Solution : Given,
Concentration of
 at equilibrium = 5.0 M
at equilibrium = 5.0 M
Concentration of
 at equilibrium = 8.0 M
at equilibrium = 8.0 M
Concentration of
 at equilibrium = 4.0 M
at equilibrium = 4.0 M
The given equilibrium reaction is,
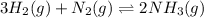
Initially a b 0
Change -3x -x +2x
At equilibrium (a-3x) (b-x) +2x
As we are given that,
Concentration of
 at equilibrium = 4.0 M = 2x
at equilibrium = 4.0 M = 2x
That means,
2x = 4.0
x = 2.0
Now we have to calculate the concentration of nitrogen gas and hydrogen gas that were reacted initially.
Concentration of
 :
:
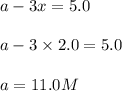
Concentration of
 :
:
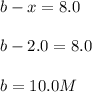
Therefore, the concentration of nitrogen gas and hydrogen gas that reacted initially are 10.0 M and 11.0 M respectively.