Mass of the mercury liquid=46593.6g
Given:
Density of the mercury liquid=13.6
Volume of the mercury liquid=3426ml
To find:
Mass of the mercury liquid
Step by Step Explanation:
Solution:
As per the formula, density of the liquid is given as
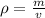 and mass can be derived as
and mass can be derived as
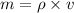
Where, m=mass of the cylinder
 =density of the cylinder
=density of the cylinder
v=volume of the cylinder
Given values are
 =13.6
=13.6
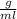 and v=3426ml
and v=3426ml
Substitute these value in the formula we get
m=13.6 \times 3426
m=46593.6g or 46.5936kg
Result:
Thus the mass of mercury liquid is 46593.6g