Answer:
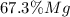
Step-by-step explanation:
Hello,
In this case, one first must consider the final pressure in order to compute the consumed hydrogen mass converted into water as shown below, considering the ideal gas equation for the gaseous water:
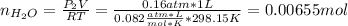
Next, taking into account the hydrogen combustion process:
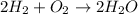
One computes the consumed hydrogen mass during as follows:
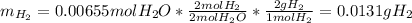
Now, such mass equals the yielded hydrogen during sample's treatment with dilute sulfuric acid:
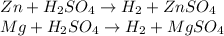
Thereby, by means of both reactions, the hydrogen mass equals:
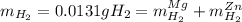
Whereas
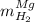 accounts for the hydrogen yielded by the magnesium and
accounts for the hydrogen yielded by the magnesium and
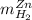 by the zinc which are computed in terms of the stoichiometry and the initial sample's composition as shown below:
by the zinc which are computed in terms of the stoichiometry and the initial sample's composition as shown below:
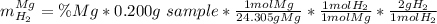
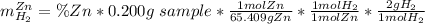
Now, as the addition between the percentages equals one, the mass of hydrogen due to zinc could be rewritten as:
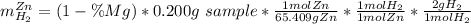
In such a way, one obtains:
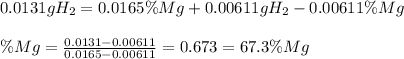
Best regards.