Answer:
77 °C
Step-by-step explanation:
This looks like a case where we can use Charles’ Law:
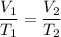
Data:
V₁ = 580 mL; T₁ = 17 °C
V₂ = 700 mL; T₂ = ?
Calculations:
(a) Convert the temperature to kelvins
T₁ = (17 + 273.15) K = 290.15 K
(b) Calculate the temperature
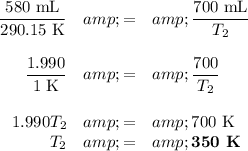
(c) Convert the temperature back to Celsius
T₂ = (350 – 273.15) °C = 77 °C