Answer:
Na = 5.911 ×
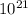 atoms/cm³
atoms/cm³
Step-by-step explanation:
given data
silver-gold alloy C Au = 17 wt%
densities ρ Au = 19.32 g/cm³
densities ρ Ag = 10.49 g/cm³
atomic weights A Au = 196.97 g/mol
atomic weights A Ag = 107.87 g/mol
solution
we will apply here formula for number of gold atoms that is
Na =
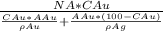 ............................1
............................1
here NA is Avogadro's number and ρ is density of two element and A is atomic weight
put here value
Na =
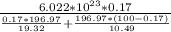
Na = 5.911 ×
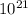 atoms/cm³
atoms/cm³