Answer:
a. 4,5x10⁻³ M
b. pH is 2,3
c. pOH is 11,7
Step-by-step explanation:
The dilution of the 0,53 M solution of the strong acid gives a concentration of:
0,53 M ×
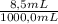 = 4,5x10⁻³ M of HCl
= 4,5x10⁻³ M of HCl
a. The H⁺ ions from HCl reacts with water as the following:
H⁺ + H₂O → H₃O⁺
Thus, molar concentration of H₃O⁺ in the diluted solution is 4,5x10⁻³ M
b. The pH is defined as -log [H₃O⁺], thus, pH of this solution is:
-log [4,5x10⁻³ M] = 2,3
c. As pH + pOH = 14, pOH is:
14- pH = 14 - 2,3 = 11,7
I hope it helps!