Answer:
40 atm
Step-by-step explanation:
- According to Boyle's law, the pressure and the volume of a fixed mass of a gas are inversely proportional at constant absolute temperature.
- That is;
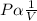
- At varying pressure and volume,
P1V1 =P2V2
In this case,
Initial volume, V1 = 2 L
Initial pressure, P1 = 1 atm
New volume, V2 = 0.05 L
We are required to calculate the new pressure,
Rearranging the formula;
P2 = P1V1 ÷ V2
= (1 atm × 2L) ÷ 0.05 L
= 40 atm
Therefore, the pressure required is 40 atm