Answer:
1.66 × 10²¹ Hz
Step-by-step explanation:
The formula for energy of light is;
E = hf , h is the plank's constant and f is the frequency
The plank's constant is 6.626 × 10⁻³⁴ J-s
We are given energy as 1.10 × 10⁻¹³ Joules
From the formula;
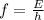
f = (1.10 × 10⁻¹³J) ÷ (6.626 × 10⁻³⁴ J-s)
= 1.66 × 10²¹ Hz
The frequency of light will be 1.66 × 10²¹ Hz