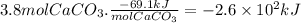Answer:
The enthalpy change is -260 kJ.
Step-by-step explanation:
Enthalpy is an extensive property, that is, it depends of the amount of matter. Let's consider the following reaction:
Ca(OH)₂(s) + CO₂(g) ⇄ CaCO₃(s) + H₂O(g)
When 1 mol of CaCO₃ is formed, 69.1 kJ of heat are released. Then, for 3.8 moles of CaCO₃,