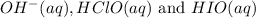Answer :
(a) The overall equation is:
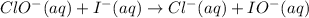
(b) The intermediates are :
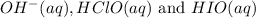
Explanation :
Part (a) :
(1)
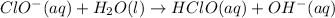 (fast)
(fast)
(2)
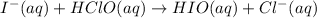 (slow)
(slow)
(3)
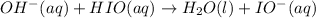 (fast)
(fast)
By adding the three equations and cancelling the common terms on both side, we will get the overall equation.
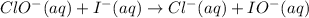
Part (b) :
Intermediates are generated and consumed in the mechanism and do not include in the overall equation.
Since, intermediates will not include in the overall mechanism.
The intermediates are :