Answer:
4Ag + O₂ → 2Ag₂O
Step-by-step explanation:
Given reaction expression:
Ag + O₂ → Ag₂O
The problem here is to balance the expression.
We are going to use a mathematical approach to solve this problem. Therefore, assign coefficients a,b and c to the species:
aAg + bO₂ → cAg₂O
Conserving Ag; a = 2c
O; 2b = c
Now let c = 1;
a = 2
b =
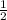
Multiply a, b and c through by 2;
a = 4, b = 1 and c = 2
4Ag + O₂ → 2Ag₂O