Answer:
Gay-Lussac’s law, because as the pressure increases, the temperature increases
Step-by-step explanation:
First of all, we can notice that the volume of the tank is fixed: this means that the volume of the air inside is also fixed.
This means that in this situation we can apply Gay-Lussac's law, which states that:
"for a gas kept at constant volume, the pressure of the gas is proportional to the absolute temperature of the gas".
Mathematically:
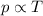
where p is the pressure in Pascal and T is the temperature in Kelvin.
In this case, the tank is filled with air: this means that the pressure of the gas inside the tank increases. And therefore, according to Gay-Lussac's law, the temperature will increase proportionally, and this explains why the tank gets hot.