Answer:
the required quantity of solution of 10% is 40 ml
the required quantity of solution of 25% = 80 ml
Step-by-step explanation:
let the required quantity of solution of 10% be x
and quantity of solution of 25% be (120 - x) ml
Hence, quantity of acid in x ml of 10 percentage solution is
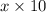 and similarly for 25% solution be
and similarly for 25% solution be
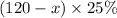
therefore total amount of acid is
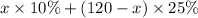
we need total solution of 120 ml of 20% so we have
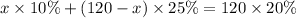
after solving we get
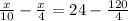
solving for x we get
x = 40
therefore ,
the required quantity of solution of 10% is 40 ml
the required quantity of solution of 25% = 80 ml