Answer: Option (C) is the correct answer.
Step-by-step explanation:
When atoms which are chemically combining tend to share electrons with each other, that is, forming covalent bonds with each other then the compound formed is known as a molecular compound.
For example,
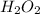 is a molecular compound.
is a molecular compound.
This is because hydrogen atom has only one electron in its valence shell and an oxygen atom needs two electrons to complete its octet.
Therefore, sharing of electrons take place between the hydrogen and oxygen atom. As a result, it is a molecular compound.
Whereas NaCl, CaO, and MgS compounds are formed due to transfer of electron from one atom to another.
Thus, we can conclude that out of the given options
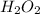 formula is a molecular compound.
formula is a molecular compound.