(a)
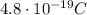
The radius of the trajectory of a charged particle moving perpendicular to a magnetic field is given by
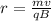
where
m is the mass of the particle
q is its charge
v is its velocity
B is the strength of the magnetic field
In this problem, we have:
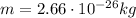
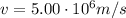
B = 1.20 T
r = 0.231 m
Solving for q, we find its charge:
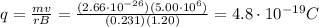
(b) 3
The charge of an electron is
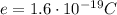
While the charge of this oxygen ion is
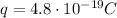
So, the ratio between the two charges is
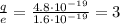
(c) Because an ion is an atom that has gained/lost an integer number of electrons
An ion is an atom that has gained/lost an integer number of electrons. In this particular case, we see that the charge of the oxygen ion is 3 times that of the electron: this means that the ion has gained/lost exactly 3 electrons.
The ratio found in part (b) cannot be a fraction, because that would mean that the atom has gained/lost a fractional number of electrons: but this is impossible, since the electron is a fundamental particle so it cannot be "divided", therefore the ratio must be an integer.