Answer: Option (a) is the correct answer.
Step-by-step explanation:
pH is the negative logarithm of concentration of hydrogen ion.
That is, pH = -log
![[H^+]](https://img.qammunity.org/2022/formulas/chemistry/high-school/869xv4va2pom353pcqwbgfhb9dbdu5d2fd.png)
Hence, pH of solution with
![[H^+] = 1 * 10^(-11)](https://img.qammunity.org/2022/formulas/chemistry/high-school/gw2yfudos7iznntt5w4ytmjpsqe44ad6yq.png) is calculated as follows.
is calculated as follows.
pH = -log
![[H^+]](https://img.qammunity.org/2022/formulas/chemistry/high-school/869xv4va2pom353pcqwbgfhb9dbdu5d2fd.png)
= -log
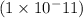
= 11
Therefore, pH of solution with
![[H^+] = 1 * 10^(-11)](https://img.qammunity.org/2022/formulas/chemistry/high-school/gw2yfudos7iznntt5w4ytmjpsqe44ad6yq.png) is 11.
is 11.