Answer:
hydrogen
Step-by-step explanation:
The combination between chlorine and hydrogen forms an explosive reaction. In fact, chlorine and hydrogen combine according to the following equation:
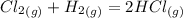
The reaction, when it takes place in a solution, that is, with water, results in fuming hydrochloric acid.