Answer:
15.85
Explanation:
If A is more acidic than B, so (pH)B > (pH)A, because -log[H⁺] decreases when [H⁺] increases, as demostrated in the graph below. So:
(pH)B - (pH)A = 1.2
-log[H⁺]B -(-log[H⁺]A) = 1.2
-log[H⁺]B + log[H⁺]A = 1.2
log([H⁺]A/[H⁺]B) = 1.2 (propertie of logaritim)
[H⁺]A/[H⁺]B =
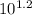
[H⁺]A/[H⁺]B = 15.85