Answer:
(a) 13.64; (b) 8.04; (c) 2.25
Step-by-step explanation:
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
![K_{\text{sp}} = {\text{[Ag$^(+)$][I$^(-)$]} = 8.3* 10^(-17)](https://img.qammunity.org/2020/formulas/chemistry/college/vaavwu3ri1jnj6td2m3n545iexsak3v4zz.png)
(a) pAg at 35.10 mL
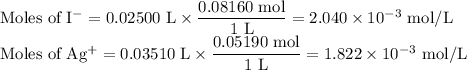
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
I/mol: 1.822 × 10⁻³ 2.040 × 10⁻³
C/mol: -1.822 × 10⁻³ -1.822 × 10⁻³
E/mol: 0 0.218 × 10⁻³
We have a saturated solution of AgI containing 0.218 × 10⁻³ mol of excess I⁻.
V = 25.00 mL + 35.10 mL = 60.10 mL
![\text{[I$^(-)$]} = \frac{0.218 * 10^(-3)\text{ mol}}{\text{0.0610 L}} = 3.57 * 10^(-3)\text{ mol/L}\\](https://img.qammunity.org/2020/formulas/chemistry/college/m6i1mi0n859fetcxw9vx6eaxmbqtpwl013.png)
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
E/mol·L⁻¹: s 3.57 × 10⁻³ + s
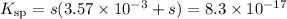
Check for negligibility:
![(3.57 * 10^(-3))/(8.3* 10^(-17)) = 4.3 * 10^(13) > 400\\\\\therefore s \ll 3.63 * 10^(-3)\\K_{\text{sp}} = s* 3.63 * 10^(-3)= 8.3* 10^(-17)\\\\s = \text{[Ag$^(+)$]} = (8.3* 10^(-17))/(3.63 * 10^(-3)) =2.29 * 10^(-14)\\\\\text{pAg} = -\log \left (2.29* 10^(-14) \right) = \mathbf{13.64}](https://img.qammunity.org/2020/formulas/chemistry/college/zkbs5gdt11xhk8eaz924o9m4hig510cns1.png)
(b) At equilibrium
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
E/mol·L⁻¹: s s
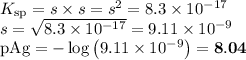
(c) At 47.10 mL
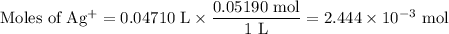
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
I/mol: 2.444 × 10⁻³ 2.040 × 10⁻³
C/mol: -2.040 × 10⁻³ -2.040 × 10⁻³
E/mol: 0.404 × 10⁻³ 0
V = 25.00 mL + 47.10 mL = 72.10 mL
![\text{[Ag$^(+)$]} = \frac{0.404 * 10^(-3)\text{ mol}}{\text{0.0721 L}} = 5.61 * 10^(-3)\text{ mol/L}\\\text{pAg} = -\log(5.61 * 10^(-3)) = \mathbf{2.25}](https://img.qammunity.org/2020/formulas/chemistry/college/luxbsc839h3l2euaal2a3rdh44pddebqmm.png)