Answer:
Pressure is increased by a factor of 2
Step-by-step explanation:
 =832 torr and
=832 torr and
 is unknown
is unknown
where
 is initial pressure and
is initial pressure and
 is the final pressure
is the final pressure
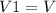 ,
,
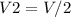 where
where
 and
and
 are the initial and final volumes respectively
are the initial and final volumes respectively
From gas laws
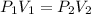
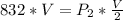
Pressure
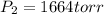
The above pressure of 1664 torr is double the pressure of 832 torr. Therefore, we conclude that the pressure is increased by a factor of 2