Answer : The correct symbol and charge for each of the ions must be
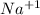 and
and
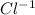 .
.
Explanation :
- Covalent compound : It is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound.
The covalent compound are usually formed when two non-metals react.
- Ionic compound : It is defined as the compound which is formed when electron gets transferred from one atom to another atom.
Ionic compound are usually formed when a metal reacts with a non-metal.
As we know that the sodium chloride is an ionic compound in which sodium is an alkali metal and has one valence electron and chlorine is a non-metal and has 7 valence electrons.
When sodium atom donates an electron to a chlorine atom then it forms an ionic compound that is sodium chloride (NaCl) and the resulting positive ion
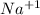 and negative ion
and negative ion
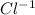 form a stable ionic compound.
form a stable ionic compound.
Hence, the correct symbol and charge for each of the ions must be
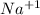 and
and
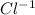 .
.