Answer:
3.99 mm
Step-by-step explanation:
To treat a diffusive process in function of time and distance we need to solve 2nd Ficks Law. This a partial differential equation, with certain condition the solution looks like this:
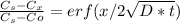
Where Cs is the concentration in the surface of the solid
Cx is the concentration at certain deep X
Co is the initial concentration of solute in the solid
and erf is the error function
First we need to solve the Cs-Cx/Cs-Co on the left to search the corresponding value later on a table.
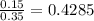
We look on a table and we see for erf(z)=0.4284 z=0.40
Then we solve for x
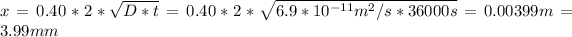 t} )[/tex]
t} )[/tex]