Step-by-step explanation:
We calculate the amount of carbon fixed as follows.
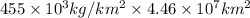
=
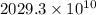 kg
kg
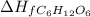 = -1273.1 kJ/mol
= -1273.1 kJ/mol
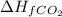 = -393.5 kJ/mol
= -393.5 kJ/mol
 = -285.8 kJ/mol
= -285.8 kJ/mol
Hence, total
 reaction will be as follows.
reaction will be as follows.
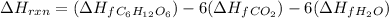
=
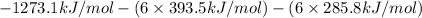
= 2802.7 kJ/mol
Therefore, calculate the number of moles of fixed carbon as follows.
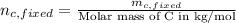
=
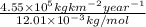
=
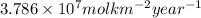
Thus, we can conclude that the annual enthalpy change resulting from the photosynthetic carbon fixation over the land surface is
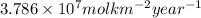 .
.