Answer:
The concentration of H₂C₂O₄ is 7,1800x10⁻³ M
Step-by-step explanation:
The reaction of the titration is:
2 KMnO₄ + H₂C₂O₄ → 2 CO₂ + K₂O + 2 MnO₃ + H₂O
22.14g of KMnO₄ in 1,00L of water have a molarity of:
22,14g KMnO₄/L×
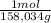 = 0,14 M of KMnO₄
= 0,14 M of KMnO₄
The moles you required for a complete reaction are:
0,14 mol KMnO₄/L× 0,02050L = 2,872x10⁻³ mol KMnO₄
By the reaction 2 moles of KMnO₄ reacts with 1 mole of H₂C₂O₄, thus, the moles of H₂C₂O₄ that react were:
2,872x10⁻³ mol KMnO₄×
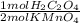 = 1,436x10⁻³ moles of H₂C₂O₄
= 1,436x10⁻³ moles of H₂C₂O₄
As the volume of the sample was 200,0mL≡ 0,2000L, The concentration of H₂C₂O₄ is:
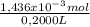 = 7,1800x10⁻³ M
= 7,1800x10⁻³ M
I hope it helps!