Answer : The value of change in entropy for melting of Hg is 9.8 J/mol.K
Explanation :
Formula used :
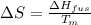
where,
 = change in entropy
= change in entropy
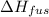 = change in enthalpy of fusion = 2.3 kJ/mol
= change in enthalpy of fusion = 2.3 kJ/mol
 = melting point temperature =
= melting point temperature =
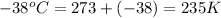
Now put all the given values in the above formula, we get:
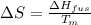
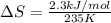
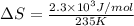
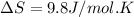
Therefore, the value of change in entropy for melting of Hg is 9.8 J/mol.K