Answer:
The formula is
![Pb(C_2H_5O_2)_2.3H_2O[tex] </p><p>Name = lead(II) hydrate trihydrate</p><p><strong>Explanation:</strong></p><p>Mass of the hydrated salt = 8.00 g</p><p>Mass of water lost = 1.14 g</p><p>Mass of the dehydrated salt = 8.00 - 1.14 g = 6.86 g </p><p>The moles of the dehydrated salt is : </p><p>Amount = 6.86 g </p><p>Molar mass of [tex]Pb(C_2H_5O_2)_2[tex] = 329.3198 g/mol</p><p>The formula for the calculation of moles is shown below: </p><p>[tex]moles = (Mass\ taken)/(Molar\ mass)]()
Thus, moles are:
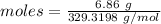
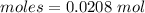
The moles of water is :
Amount = 1.14 g
Molar mass of
![H_2O[tex] = 18 g/mol </p><p>The formula for the calculation of moles is shown below: </p><p>[tex]moles = (Mass\ taken)/(Molar\ mass)](https://img.qammunity.org/2020/formulas/chemistry/college/qljmwkml3xmvota8uobancvnwmiyspp2mh.png)
Thus, moles are:
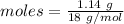
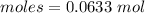
The simplest ration of the two are:
[tex]Pb(C_2H_5O_2)_2:H_2O[tex] = 0.0208 : 0.0633 = 1 : 3
The formula is [tex]Pb(C_2H_5O_2)_2.3H_2O[tex]
Name = lead(II) hydrate trihydrate