Answer:
29Si has a natural abundance of 4.68%.
30Si has a relative atomic mass of 29.99288 and a natural abundance of 3.09%.
Step-by-step explanation:
The atomic mass of silicon is given by:
Si=Si²⁸×A₁+Si²⁹×A₂+Si³⁰×A₃
Where:
Si: atomic mass of silicon (28.086)
Si²⁸: relative atomic mass of 28Si (27.97693)
A₁: natural abundance of 28Si (92.23%)
Si²⁹: relative atomic mass of 29Si (28.97649)
A₂: natural abundance of 29Si
Si³⁰: relative atomic mass of 30Si
A₃: natural abundance of 30Si
We also know that 30Si natural abundance is in the ratio of 0.6592 to that of 29Si.
We have to set up a system of three equations in three unknowns:
Si=Si²⁸×A₁+Si²⁹×A₂+Si³⁰×A₃
A₃=0.6592×A₂
A₁+A₂+A₃=1
First, we find substitute the value of A₃ in the third equation and solv teh value of A₂:
A₁+A₂+0.6592×A₂=1
A₁+1.6592×A₂=1
1.6592×A₂=1-A₁
A₂=
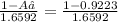 =0.0468
=0.0468
Then, we find the value of A₃:
A₃=0.6592×A₂
A₃=0.6592×0.0468=0.0309
Finally, we find the value of Si³⁰ in the first equation:
Si=Si²⁸×A₁+Si²⁹×A₂+Si³⁰×A₃
28.086=27.97693×0.9223+28.97649×0.0468+Si³⁰×0.0309
28.086=27.15922+Si³⁰×0.0309
28.086-27.15922=Si³⁰×0.0309
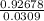 =Si³⁰
=Si³⁰
Si³⁰=29.99288