Pressure of the ideal gas=505.7kPa
Given:
No of moles=0.907mole
Temperature of the gas=
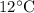
Volume of the gas=4.25L
To find:
Pressure of the gas
Step by Step Explanation:
Solution:
According to the ideal gas equation
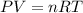 and from this pressure is derived as
and from this pressure is derived as
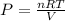
Where P=Pressure of the gas
V=Volume of the gas=4.25L
n=No of the moles=0.907mole
R=Gas constant=8.314
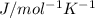
T=Temperature of the gas=
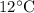 =273+12=285K
=273+12=285K
Substitute these known values in the above equation we get
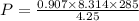
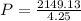
P=505.7kPa
Result:
Thus the pressure of the ideal gas is 505.7kPa