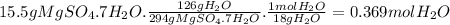Answer:
0.369 moles of H₂O.
Step-by-step explanation:
The molar mass of the hydrate salt is equal to the molar mass of the anhydrous salt plus the molar mass of water times the moles of water.
M(MgSO₄·7H₂O) = M(MgSO₄) + 7 × M(H₂O)
M(MgSO₄·7H₂O) = 168 g/mol + 7 × 18 g/mol
M(MgSO₄·7H₂O) = 294 g/mol
Every 294 g of MgSO₄·7H₂O there are 126 g of H₂O. So, for 15.5 g of MgSO₄·7H₂O,