Answer:
wavelength of the second photon emitted is 97.26 nm
Step-by-step explanation:
Data provided;
Wavelength absorbed = 94.98 nm
Wavelength of the one of the emitted photon = 4052.3 nm
Now,
The energy is given as:
Energy =
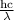
here,
h is the plank's constant
c is the speed of the light
λ is the wavelength
Now,
by the principle of conservation of energy
Initial energy = Final energy
Therefore,
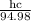 =
=
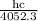 +
+
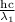
or
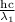 =
=
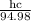 -
-
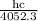
or
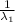 =
=
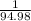 -
-
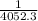
or
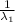 = 0.0105 - 2.46 × 10⁻⁴
= 0.0105 - 2.46 × 10⁻⁴
or
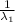 = 0.01028
= 0.01028
or
λ₁ = 97.26 nm
Hence,
the wavelength of the second photon emitted is 97.26 nm